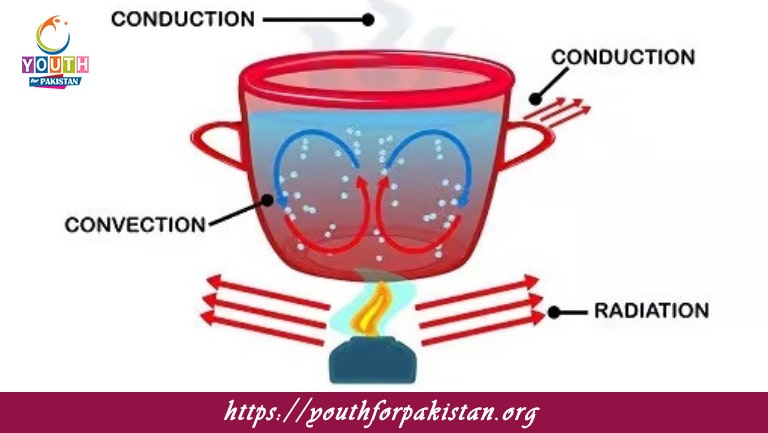
Welcome to the Thermodynamics MDCAT MCQs with Answers. In this post, we have shared Thermodynamics Multiple Choice Questions and Answers for PMC MDCAT 2024. Each question in MDCAT Physics offers a chance to enhance your knowledge regarding Thermodynamics MCQs in this MDCAT Online Test.
Thermodynamics MDCAT MCQs Test Preparations
The first law of thermodynamics is a restatement of the law of:
a) Conservation of energy
b) Conservation of mass
c) Conservation of momentum
d) Conservation of charge
The unit of entropy is:
a) Joule
b) Kelvin
c) Joule per Kelvin
d) Newton
A process in which no heat is exchanged with the surroundings is called:
a) Isothermal
b) Adiabatic
c) Isobaric
d) Isochoric
The measure of disorder or randomness of a system is called:
a) Enthalpy
b) Entropy
c) Internal energy
d) Gibbs free energy
The efficiency of a Carnot engine depends on:
a) The nature of the working substance
b) The pressure of the system
c) The temperature difference between the hot and cold reservoirs
d) The volume of the working substance
The heat capacity at constant volume is represented by:
a) Cv
b) Cp
c) Q
d) W
The specific heat capacity of water is:
a) 1 J/g°C
b) 4.18 J/g°C
c) 0.5 J/g°C
d) 2.09 J/g°C
In an isothermal process, the internal energy of an ideal gas:
a) Increases
b) Decreases
c) Remains constant
d) First increases, then decreases
For an ideal gas, the internal energy depends only on:
a) Volume
b) Pressure
c) Temperature
d) Entropy
The Zeroth law of thermodynamics is related to:
a) Conservation of energy
b) Thermal equilibrium
c) Entropy
d) Enthalpy
The amount of heat required to raise the temperature of a substance by one degree Celsius is called:
a) Latent heat
b) Specific heat
c) Heat capacity
d) Thermal conductivity
The change in enthalpy at constant pressure is equal to:
a) Work done
b) Heat absorbed or released
c) Internal energy change
d) Temperature change
A process that occurs without any change in enthalpy is called:
a) Adiabatic
b) Isochoric
c) Isobaric
d) Isenthalpic
The second law of thermodynamics states that:
a) Energy can neither be created nor destroyed
b) The total entropy of an isolated system can never decrease
c) The internal energy of an isolated system is constant
d) Heat flows from a colder to a hotter body
In a heat engine, the work done by the system is equal to:
a) The heat absorbed by the system
b) The difference between heat absorbed and heat rejected
c) The sum of heat absorbed and heat rejected
d) The heat rejected by the system
The triple point of water is the temperature and pressure at which:
a) Water boils
b) Ice melts
c) Water, ice, and vapor coexist in equilibrium
d) Water freezes
The internal energy of an ideal gas is a function of:
a) Volume
b) Temperature
c) Pressure
d) Entropy
The work done in a reversible adiabatic process is:
a) Positive
b) Negative
c) Zero
d) Independent of the process
The Carnot cycle consists of:
a) Two isothermal and two adiabatic processes
b) Two isobaric and two isochoric processes
c) Two adiabatic and two isobaric processes
d) Two isochoric and two isothermal processes
The coefficient of performance (COP) of a refrigerator is defined as:
a) The ratio of work input to the heat extracted from the cold reservoir
b) The ratio of heat extracted from the cold reservoir to work input
c) The ratio of work input to the heat rejected to the hot reservoir
d) The ratio of heat rejected to the hot reservoir to work input
An ideal gas undergoes an isothermal expansion at 300K. If the initial pressure is 2 atm and the final pressure is 1 atm, the work done by the gas is:
a) 0 J
b) 8314 J
c) 2078 J
d) 4140 J
The enthalpy change for a reaction is negative. The reaction is:
a) Exothermic
b) Endothermic
c) Isothermal
d) Adiabatic
The work done in an isochoric process is:
a) Positive
b) Negative
c) Zero
d) Maximum
The entropy change of the universe for a spontaneous process is:
a) Positive
b) Negative
c) Zero
d) Depends on the process
The maximum efficiency of a heat engine operating between a hot reservoir at 500 K and a cold reservoir at 300 K is:
a) 40%
b) 50%
c) 60%
d) 70%
The thermodynamic process in which temperature remains constant is called:
a) Isobaric
b) Isochoric
c) Isothermal
d) Adiabatic
The heat absorbed by a system at constant pressure is equal to its:
a) Internal energy change
b) Entropy change
c) Enthalpy change
d) Temperature change
The Clausius statement of the second law of thermodynamics states that:
a) Heat can spontaneously flow from a colder to a hotter body
b) Heat cannot spontaneously flow from a colder to a hotter body
c) Work can be converted entirely into heat
d) Heat can be converted entirely into work
The maximum work obtained from a heat engine is called:
a) Mechanical work
b) Useful work
c) Net work
d) Available work
For an ideal gas, the work done in an isothermal process is proportional to:
a) Temperature
b) Pressure
c) Volume
d) Logarithm of the volume ratio
The temperature at which the entropy of a perfect crystal becomes zero is:
a) 0°C
b) 273.15 K
c) -273.15°C
d) 0 K
The Helmholtz free energy is a measure of:
a) The maximum useful work obtainable from a system at constant temperature and volume
b) The maximum useful work obtainable from a system at constant temperature and pressure
c) The internal energy of a system
d) The enthalpy of a system
The term “thermal conductivity” refers to:
a) The amount of heat required to change the temperature of a substance
b) The ability of a substance to conduct heat
c) The heat absorbed or released during a phase change
d) The resistance to heat flow
The phase of a substance with the highest entropy is:
a) Solid
b) Liquid
c) Gas
d) Plasma
The temperature of an ideal gas is directly proportional to:
a) The pressure at constant volume
b) The volume at constant pressure
c) The number of moles at constant pressure
d) The internal energy at constant volume
The term “enthalpy” is defined as:
a) The total energy of a system
b) The heat content of a system
c) The work done by a system
d) The change in temperature of a system
In a reversible process, the total entropy change of the system and surroundings is:
a) Positive
b) Negative
c) Zero
d) Infinite
The specific heat capacity at constant pressure (Cp) is always:
a) Less than Cv
b) Equal to Cv
c) Greater than Cv
d) Independent of Cv
The term “latent heat” refers to:
a) The heat absorbed or released during a temperature change
b) The heat absorbed or released during a phase change
c) The heat absorbed or released during a chemical reaction
d) The heat absorbed or released during an isothermal process
The Van der Waals equation accounts for:
a) Ideal gas behavior
b) Non-ideal gas behavior
c) Solid-state behavior
d) Liquid-state behavior
If you are interested to enhance your knowledge regarding Physics, Chemistry, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.