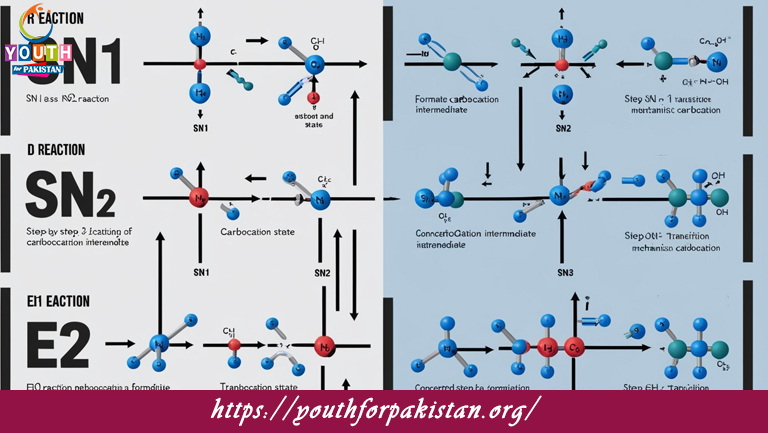
Welcome to the Sn1, Sn2, E1 And E2 Reaction MDCAT MCQs with Answers. In this post, we have shared Sn1, Sn2, E1 And E2 Reaction Multiple Choice Questions and Answers for PMC MDCAT 2024. Each question in MDCAT Chemistry offers a chance to enhance your knowledge regarding Sn1, Sn2, E1 And E2 Reaction MCQs in this MDCAT Online Test.
Which of the following mechanisms involves a carbocation intermediate?
a) SN2
b) E2
c) SN1
d) E1
In which mechanism does the rate-determining step involve a nucleophile attacking the substrate?
a) SN1
b) E1
c) SN2
d) E2
Which of the following reactions typically proceeds via an E1 mechanism?
a) Dehydrohalogenation of a primary alkyl halide
b) Dehydrohalogenation of a tertiary alkyl halide
c) Nucleophilic substitution of a primary alkyl halide
d) Nucleophilic substitution of a tertiary alkyl halide
What type of reaction does not proceed via a carbocation intermediate?
a) E1
b) SN1
c) SN2
d) Both E1 and SN1
In which mechanism is the reaction rate dependent on both the substrate and the nucleophile?
a) SN1
b) E1
c) SN2
d) E2
Which reaction mechanism typically involves a strong base?
a) SN1
b) E1
c) SN2
d) E2
Which of the following reactions usually occurs in a polar protic solvent?
a) SN2
b) E2
c) E1
d) SN1
The formation of a carbocation intermediate is a characteristic feature of:
a) SN2 reaction
b) E2 reaction
c) E1 reaction
d) All of the above
Which reaction is most favored by a strong nucleophile?
a) SN1
b) E1
c) SN2
d) E2
In which type of reaction is the stereochemistry of the substrate retained in the product?
a) SN1
b) SN2
c) E1
d) E2
Which reaction typically occurs with a tertiary alkyl halide and a weak nucleophile/base?
a) SN2
b) E2
c) SN1
d) E1
The reaction of an alkyl halide with a strong base and a good leaving group is usually:
a) E1
b) E2
c) SN1
d) SN2
In which mechanism does the nucleophile attack the substrate from the opposite side of the leaving group?
a) SN1
b) E1
c) SN2
d) E2
Which of the following is an example of a strong nucleophile?
a) Water
b) Methanol
c) Sodium hydroxide
d) Ethanol
Which reaction mechanism is favored by a bulky base?
a) SN1
b) E1
c) SN2
d) E2
In the E1 mechanism, the formation of the carbocation occurs in which step?
a) First step
b) Second step
c) Third step
d) Fourth step
Which reaction mechanism is most likely to occur with a primary alkyl halide?
a) SN1
b) E1
c) SN2
d) E2
Which of the following conditions favors an E2 reaction over an SN2 reaction?
a) Strong nucleophile
b) Weak base
c) Polar aprotic solvent
d) Steric hindrance
Which reaction involves the formation of an intermediate with a positive charge?
a) SN2
b) E2
c) E1
d) SN1
In the SN2 mechanism, the rate of reaction depends on:
a) The concentration of the substrate only
b) The concentration of the nucleophile only
c) Both the nucleophile and the substrate
d) Neither the nucleophile nor the substrate
Which of the following mechanisms requires the presence of a strong acid catalyst?
a) SN1
b) E1
c) E2
d) SN2
In the E2 reaction, the base removes a proton from which type of carbon?
a) Primary
b) Secondary
c) Tertiary
d) Carbon adjacent to the leaving group
The rate of an SN1 reaction depends on the concentration of:
a) The nucleophile
b) The substrate
c) Both the nucleophile and the substrate
d) Neither the nucleophile nor the substrate
Which reaction mechanism is characterized by a single concerted step?
a) E1
b) SN1
c) SN2
d) E2
The formation of a double bond during elimination reactions occurs in which mechanism?
a) SN1
b) E1
c) SN2
d) Both E1 and E2
Which reaction mechanism involves a step where the leaving group departs before the nucleophile attacks?
a) SN2
b) E1
c) E2
d) Both SN1 and E2
The SN2 reaction is characterized by:
a) Inversion of stereochemistry
b) Formation of a carbocation
c) Formation of a racemic mixture
d) The leaving group departing first
Which reaction mechanism is not favored by a polar protic solvent?
a) SN1
b) E1
c) SN2
d) E2
In which mechanism is the reaction rate independent of the concentration of the nucleophile?
a) E2
b) E1
c) SN1
d) SN2
The reaction of an alkyl halide with a strong base to form an alkene is an example of:
a) E1
b) SN2
c) E2
d) SN1
Which type of alcohol would most likely undergo an E2 reaction?
a) Primary
b) Secondary
c) Tertiary
d) None
What type of mechanism is favored by a substrate with a good leaving group and a weak nucleophile?
a) E1
b) E2
c) SN2
d) SN1
Which mechanism is favored by a good nucleophile and a strong base?
a) E1
b) E2
c) SN2
d) SN1
Which reaction mechanism involves a transition state with partial bonds?
a) E2
b) E1
c) SN1
d) SN2
In which mechanism does the nucleophile and leaving group both participate in the rate-determining step?
a) E1
b) E2
c) SN1
d) SN2
Which reaction mechanism is characterized by the formation of a double bond in the product?
a) SN2
b) SN1
c) E1
d) None
Which type of reaction is likely to occur with a tertiary alkyl halide and a strong nucleophile?
a) SN2
b) E2
c) SN1
d) E1
The reaction of an alkyl halide with a weak base usually results in:
a) SN2
b) E2
c) E1
d) SN1
Which of the following conditions is least likely to favor an SN1 reaction?
a) Weak nucleophile
b) Polar protic solvent
c) Tertiary substrate
d) Strong base
The product of an E2 reaction typically involves:
a) Formation of a new carbon-carbon single bond
b) Formation of a carbon-carbon double bond
c) Formation of a carbocation
d) Formation of a racemic mixture
If you are interested to enhance your knowledge regarding Physics, Chemistry, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.