Welcome to the Charles‟S Law MDCAT MCQs with Answers. In this post, we have shared Charles‟S Law Multiple Choice Questions and Answers for PMC MDCAT 2024. Each question in MDCAT Chemistry offers a chance to enhance your knowledge regarding Charles‟S Law MCQs in this MDCAT Online Test.
Charles’s Law states that the volume of a gas is directly proportional to its temperature when pressure is constant. This can be expressed as:
a) V ∝ T
b) V ∝ 1/T
c) V ∝ P/T
d) V ∝ T/P
If the temperature of a gas increases from 300 K to 600 K while the pressure remains constant, what happens to the volume of the gas?
a) It decreases
b) It doubles
c) It triples
d) It remains the same
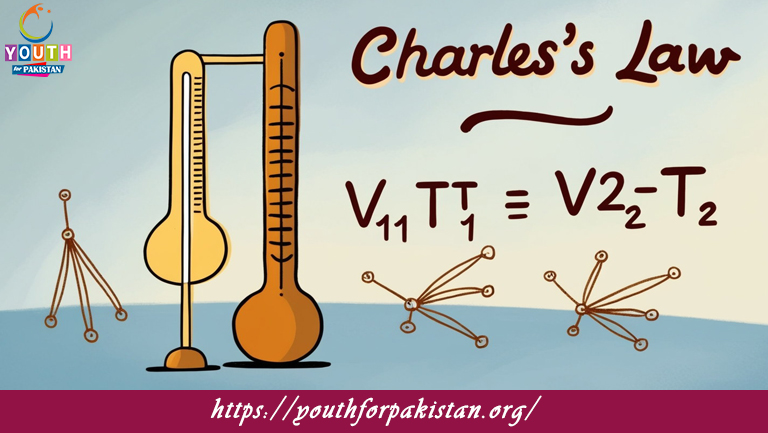
In Charles’s Law, if the initial volume and temperature of a gas are V₁ and T₁ respectively, and the final volume and temperature are V₂ and T₂ respectively, the law can be mathematically expressed as:
a) V₁/T₁ = V₂/T₂
b) V₁/T₁ = T₂/V₂
c) V₂/T₂ = V₁/T₂
d) V₁T₂ = V₂T₁
At constant pressure, what happens to the volume of a gas if its temperature is reduced by half?
a) The volume is reduced by half
b) The volume remains the same
c) The volume is doubled
d) The volume is quadrupled
If a gas occupies a volume of 4 L at 300 K, what will be its volume at 600 K if pressure is constant?
a) 2 L
b) 4 L
c) 6 L
d) 8 L
Charles’s Law is valid for:
a) All gases
b) Only ideal gases
c) Only real gases
d) Only gases at high pressures
What is the temperature at which the volume of a gas would theoretically become zero according to Charles’s Law?
a) 0°C
b) -273°C
c) 273 K
d) 0 K
If a gas at 200 K has a volume of 5 L, what will be the volume of the gas at 400 K, assuming constant pressure?
a) 2.5 L
b) 5 L
c) 10 L
d) 20 L
When using Charles’s Law, temperatures must be in:
a) Celsius
b) Fahrenheit
c) Kelvin
d) Rankine
According to Charles’s Law, if the volume of a gas is 3 L at 350 K, what will be its volume at 700 K?
a) 1.5 L
b) 3 L
c) 4.5 L
d) 7 L
Which of the following is an example of Charles’s Law in real life?
a) A balloon expanding in hot air
b) A balloon contracting when taken to high altitudes
c) The compressibility of gases at high pressure
d) The solubility of gases in liquids
If a gas occupies 10 L at 50°C, what will be its volume at 100°C, assuming constant pressure?
a) 5 L
b) 10 L
c) 15 L
d) 20 L
Charles’s Law is most applicable to which type of gases?
a) Real gases at high pressure
b) Ideal gases at high temperature
c) Real gases at low temperature
d) Ideal gases at low pressure
Which of the following statements is true according to Charles’s Law?
a) Volume increases as temperature decreases
b) Volume decreases as temperature increases
c) Volume is independent of temperature
d) Volume increases as temperature increases
If the temperature of a gas is decreased to one-fourth of its original value, what happens to the volume, assuming constant pressure?
a) It remains the same
b) It is reduced to one-fourth
c) It is doubled
d) It is quadrupled
In a given experiment, a gas at 500 K has a volume of 8 L. What will be its volume at 250 K if pressure is constant?
a) 4 L
b) 8 L
c) 16 L
d) 32 L
When the temperature of a gas is increased, what happens to the kinetic energy of the gas molecules?
a) It decreases
b) It remains the same
c) It increases
d) It becomes zero
What is the relationship between volume and temperature in Charles’s Law?
a) Inversely proportional
b) Directly proportional
c) Independent
d) Exponentially proportional
At which temperature does the gas volume become zero according to Charles’s Law?
a) 0°C
b) -273°C
c) 273 K
d) 0 K
If a gas sample is cooled from 500 K to 250 K and its initial volume is 12 L, what is its final volume?
a) 6 L
b) 12 L
c) 24 L
d) 48 L
Charles’s Law can be derived from:
a) Boyle’s Law
b) Avogadro’s Law
c) Ideal Gas Law
d) Dalton’s Law
If the temperature of a gas is raised, the volume of the gas will:
a) Increase if pressure is constant
b) Decrease if pressure is constant
c) Remain the same
d) Decrease if temperature is increased
What is the initial volume of a gas if its volume at 500 K is 20 L and its volume at 400 K is 16 L?
a) 12 L
b) 18 L
c) 22 L
d) 24 L
In which conditions does Charles’s Law apply?
a) High pressure
b) Low temperature
c) Constant pressure
d) Variable volume
If the volume of a gas is 8 L at 200°C, what will be the volume at 100°C if pressure remains constant?
a) 4 L
b) 6 L
c) 8 L
d) 12 L
What happens to the volume of a gas when it is heated from 20°C to 40°C, assuming constant pressure?
a) It decreases
b) It remains the same
c) It increases
d) It doubles
Which gas law is combined with Charles’s Law in the Ideal Gas Law?
a) Boyle’s Law
b) Avogadro’s Law
c) Gay-Lussac’s Law
d) Dalton’s Law
In Charles’s Law, if the volume of a gas is doubled, the temperature in Kelvin must:
a) Decrease
b) Remain the same
c) Increase
d) Be halved
Which of the following graphs represents Charles’s Law?
a) Volume vs. Pressure
b) Volume vs. Temperature
c) Pressure vs. Temperature
d) Volume vs. Number of moles
The temperature of a gas is increased from 100 K to 200 K. If the initial volume was 5 L, what will be the final volume?
a) 2.5 L
b) 5 L
c) 7.5 L
d) 10 L
In Charles’s Law, the temperature must always be in:
a) Celsius
b) Fahrenheit
c) Kelvin
d) Rankine
If the temperature of a gas increases, the volume of the gas will:
a) Increase if pressure is held constant
b) Decrease if pressure is held constant
c) Remain the same
d) First increase, then decrease
A gas with a volume of 15 L at 300 K is cooled to 150 K. What will be its new volume?
a) 7.5 L
b) 15 L
c) 22.5 L
d) 30 L
Charles’s Law is a special case of which gas law?
a) Boyle’s Law
b) Ideal Gas Law
c) Avogadro’s Law
d) Gay-Lussac’s Law
If a gas expands from 4 L to 8 L, what happens to its temperature, assuming constant pressure?
a) The temperature remains the same
b) The temperature doubles
c) The temperature is halved
d) The temperature is reduced to one-fourth
At what temperature will the volume of a gas theoretically become zero according to Charles’s Law?
a) -273°C
b) 0°C
c) 100 K
d) 273 K
If the temperature of a gas is increased by 50%, what is the expected change in volume?
a) Volume decreases by 50%
b) Volume increases by 50%
c) Volume remains the same
d) Volume doubles
Which graph best represents Charles’s Law?
a) Volume vs. Pressure
b) Volume vs. Temperature
c) Temperature vs. Pressure
d) Volume vs. Moles
If a gas at 600 K has a volume of 10 L, what will be its volume at 300 K?
a) 5 L
b) 10 L
c) 20 L
d) 30 L
In Charles’s Law, what happens to the volume of a gas when its temperature is increased from 250 K to 500 K, keeping pressure constant?
a) Volume decreases
b) Volume remains the same
c) Volume doubles
d) Volume quadruples
If you are interested to enhance your knowledge regarding Physics, Chemistry, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.