Welcome to the Atomic Spectra MDCAT MCQs with Answers. In this post, we have shared Atomic Spectra Multiple Choice Questions and Answers for PMC MDCAT 2024. Each question in MDCAT Physics offers a chance to enhance your knowledge regarding Atomic Spectra MCQs in this MDCAT Online Test.
Atomic Spectra MDCAT MCQs Test Preparations
What is the name given to the series of lines in the hydrogen spectrum corresponding to transitions from higher energy levels to the first energy level?
A) Balmer series
B) Lyman series
C) Paschen series
D) Brackett series
The spectral lines observed in the visible region of the hydrogen spectrum are known as:
A) Balmer series
B) Lyman series
C) Paschen series
D) Pfund series
Which scientist proposed the quantization of electron orbits in the hydrogen atom?
A) Albert Einstein
B) Niels Bohr
C) Werner Heisenberg
D) Louis de Broglie
What is the principal quantum number of the ground state of a hydrogen atom?
A) 0
B) 1
C) 2
D) 3
The Rydberg constant is a fundamental constant in atomic physics. Its value is closest to:
A) 1.097 x 10^7 m^-1
B) 3.00 x 10^8 m^-1
C) 6.626 x 10^-34 m^-1
D) 9.109 x 10^-31 m^-1
The emission spectrum of hydrogen in the ultraviolet region is known as:
A) Balmer series
B) Lyman series
C) Paschen series
D) Pfund series
Which of the following transitions in the hydrogen atom would result in the emission of a photon with the highest energy?
A) n=3 to n=2
B) n=4 to n=3
C) n=5 to n=4
D) n=2 to n=1
The Balmer series of spectral lines is observed in which region of the electromagnetic spectrum?
A) Ultraviolet
B) Visible
C) Infrared
D) Microwave
Which series of lines in the hydrogen spectrum corresponds to transitions from higher energy levels to the third energy level?
A) Balmer series
B) Lyman series
C) Paschen series
D) Pfund series
In the context of atomic spectra, the term “spectral line” refers to:
A) A continuous range of wavelengths
B) A specific wavelength or frequency
C) The visible part of the spectrum
D) The entire electromagnetic spectrum
The phenomenon of splitting of spectral lines in the presence of a magnetic field is called:
A) Zeeman effect
B) Stark effect
C) Raman effect
D) Doppler effect
The Paschen series of hydrogen spectral lines is observed in which region of the electromagnetic spectrum?
A) Ultraviolet
B) Visible
C) Infrared
D) X-ray
The energy of a photon emitted during an electronic transition is given by:
A) E = mc^2
B) E = hν
C) E = 1/2 mv^2
D) E = kT
The difference in energy between two energy levels in an atom is known as:
A) Work function
B) Ionization energy
C) Energy gap
D) Photon energy
In the hydrogen atom, which series corresponds to transitions that end at the fourth energy level?
A) Balmer series
B) Lyman series
C) Paschen series
D) Brackett series
Which quantum number determines the shape of an electron’s orbital?
A) Principal quantum number
B) Azimuthal quantum number
C) Magnetic quantum number
D) Spin quantum number
The transition from n=3 to n=2 in the hydrogen atom emits a photon in which part of the spectrum?
A) Ultraviolet
B) Visible
C) Infrared
D) Microwave
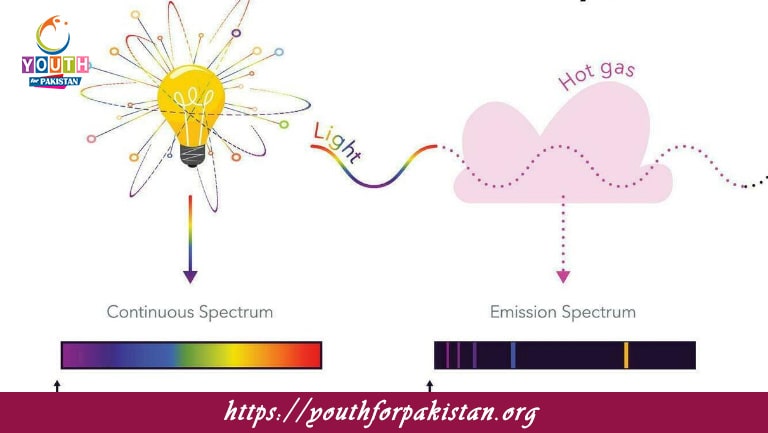
What is the primary difference between absorption and emission spectra?
A) Absorption spectra show dark lines on a bright background, while emission spectra show bright lines on a dark background.
B) Absorption spectra show bright lines on a dark background, while emission spectra show dark lines on a bright background.
C) Both spectra are identical.
D) Absorption spectra only occur in the visible region, while emission spectra occur in the infrared region.
The hydrogen atom emits a photon with a wavelength of 121.6 nm. To which series does this transition belong?
A) Lyman series
B) Balmer series
C) Paschen series
D) Brackett series
What is the highest energy level (n) involved in the Balmer series transitions?
A) 1
B) 2
C) 3
D) Infinity
Which of the following is the correct expression for the wavelength of spectral lines in the hydrogen atom?
A) λ = RZ^2(1/n1^2 – 1/n2^2)
B) λ = R(1/n1^2 – 1/n2^2)
C) 1/λ = R(1/n1^2 – 1/n2^2)
D) 1/λ = RZ^2(1/n1^2 – 1/n2^2)
Which of the following quantum numbers describes the orientation of an electron’s orbital?
A) Principal quantum number
B) Azimuthal quantum number
C) Magnetic quantum number
D) Spin quantum number
The shortest wavelength transition in the hydrogen atom corresponds to which series?
A) Balmer series
B) Lyman series
C) Paschen series
D) Brackett series
The quantum mechanical model of the atom was developed by which scientist?
A) Niels Bohr
B) Werner Heisenberg
C) Erwin Schrödinger
D) Albert Einstein
Which effect explains the splitting of spectral lines due to an electric field?
A) Zeeman effect
B) Stark effect
C) Raman effect
D) Doppler effect
The energy levels of an electron in a hydrogen atom are given by which formula?
A) E = mc^2
B) E = -13.6 eV/n^2
C) E = hν
D) E = 1/2 mv^2
In a hydrogen atom, the energy difference between the n=1 and n=2 levels is:
A) 10.2 eV
B) 12.1 eV
C) 13.6 eV
D) 14.2 eV
The lines in the hydrogen spectrum are produced by:
A) Electrons falling to higher energy levels
B) Electrons jumping to higher energy levels
C) Electrons jumping to lower energy levels
D) Protons moving between energy levels
Which of the following is a possible transition in the hydrogen atom?
A) n=2 to n=3
B) n=1 to n=1
C) n=4 to n=5
D) n=3 to n=2
The frequency of light emitted or absorbed during an electronic transition in an atom is directly proportional to:
A) The velocity of the electron
B) The wavelength of the light
C) The energy difference between the initial and final states
D) The distance between the nucleus and the electron
The first line of the Balmer series corresponds to the transition from:
A) n=2 to n=1
B) n=3 to n=2
C) n=4 to n=3
D) n=5 to n=4
In the hydrogen atom, the second line of the Balmer series is known as:
A) Hα
B) Hβ
C) Hγ
D) Hδ
The spectral series of hydrogen that lies in the infrared region is called:
A) Lyman series
B) Balmer series
C) Paschen series
D) Brackett series
The term “fine structure” in atomic spectra refers to:
A) The splitting of spectral lines due to electron spin
B) The splitting of spectral lines due to an external magnetic field
C) The broadening of spectral lines due to temperature effects
D) The shifting of spectral lines due to the Doppler effect
The transition from n=4 to n=3 in the hydrogen atom emits a photon in which part of the spectrum?
A) Ultraviolet
B) Visible
C) Infrared
D) Microwave
What is the wavelength of the Hα line in the Balmer series?
A) 121.6 nm
B) 434.0 nm
C) 486.1 nm
D) 656.3 nm
The Heisenberg uncertainty principle is associated with:
A) The wave nature of particles
B) The particle nature of light
C) The impossibility of knowing both the position and momentum of an electron simultaneously
D) The quantization of energy levels
The principle that no two electrons in an atom can have the same set of quantum numbers is known as:
A) Hund’s rule
B) Pauli exclusion principle
C) Aufbau principle
D) Bohr’s postulate
Which of the following transitions in the hydrogen atom would result in the absorption of a photon with the lowest energy?
A) n=1 to n=2
B) n=2 to n=3
C) n=3 to n=4
D) n=4 to n=5
The concept of wave-particle duality is associated with which scientist?
A) Albert Einstein
B) Niels Bohr
C) Louis de Broglie
D) Werner Heisenberg
If you are interested to enhance your knowledge regarding Physics, Chemistry, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.