Welcome to the Coulomb’s Law MDCAT MCQs with Answers. In this post, we have shared Coulomb’s Law Multiple Choice Questions and Answers for PMC MDCAT 2024. Each question in MDCAT Physics offers a chance to enhance your knowledge regarding Coulomb’s Law MCQs in this MDCAT Online Test.
Coulomb’s Law MDCAT MCQs Test Preparations
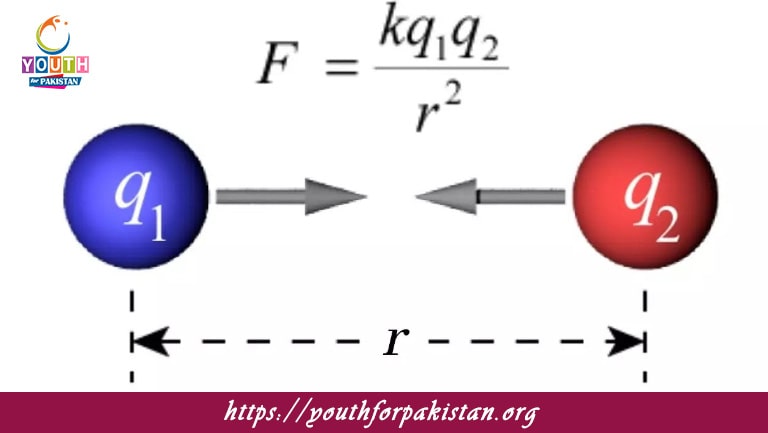
Coulomb’s law states that the force between two point charges is directly proportional to:
a) The distance between the charges
b) The product of the magnitudes of the charges
c) The sum of the charges
d) The square of the distance between the charges
The force between two point charges is inversely proportional to:
a) The distance between them
b) The square of the distance between them
c) The product of their charges
d) The sum of their charges
The constant of proportionality in Coulomb’s law in a vacuum is:
a) 9 × 10^9 Nm²/C²
b) 8.85 × 10^-12 F/m
c) 1 × 10^-7 Tm/A
d) 3 × 10^8 m/s
If the distance between two charges is doubled, the force between them becomes:
a) Four times as great
b) Half as great
c) One-fourth as great
d) Twice as great
The force between two charges is maximum when the charges are:
a) Large and far apart
b) Small and close together
c) Large and close together
d) Small and far apart
Coulomb’s law can be used to calculate the force between:
a) Two charged spheres
b) Two charged plates
c) Two point charges
d) A point charge and a neutral object
Coulomb’s law is valid for:
a) Only point charges
b) Only spherical charges
c) Both point and spherical charges
d) Any shape of charges
The force between two charges in a medium with dielectric constant εr is:
a) εr times the force in vacuum
b) (1/εr) times the force in vacuum
c) (1/εr²) times the force in vacuum
d) εr² times the force in vacuum
The SI unit of charge is:
a) Newton
b) Joule
c) Coulomb
d) Volt
The direction of the force between two like charges is:
a) Attractive
b) Repulsive
c) Parallel
d) Perpendicular
The force between two charges is zero when:
a) The charges are equal
b) The charges are of opposite sign
c) The distance between them is infinite
d) The charges are at the same point
In Coulomb’s law, the force is proportional to:
a) The sum of the charges
b) The square of the sum of the charges
c) The product of the charges
d) The difference in the charges
Coulomb’s law describes the force between:
a) Electric currents
b) Magnetic poles
c) Stationary charges
d) Moving charges
The force between two charges separated by a vacuum depends on:
a) The medium between them
b) The distance between them
c) The speed of light
d) The direction of the force
If the charge of one of the particles is doubled, the force between two charges:
a) Remains the same
b) Doubles
c) Halves
d) Quadruples
The electrostatic force between two charges is inversely proportional to:
a) The sum of their magnitudes
b) The square of the distance between them
c) The difference in their magnitudes
d) The square of the sum of their magnitudes
The force between two charges is independent of:
a) The nature of the medium between them
b) The product of the charges
c) The distance between them
d) The presence of other charges
The electrostatic force between two charges is:
a) Always attractive
b) Always repulsive
c) Either attractive or repulsive
d) Neither attractive nor repulsive
The force between two charges is strongest when:
a) The charges are small
b) The distance between them is small
c) The charges are far apart
d) The medium between them has a high dielectric constant
Coulomb’s law constant (k) in a vacuum is approximately:
a) 9 × 10^9 Nm²/C²
b) 8.85 × 10^-12 F/m
c) 1 × 10^-7 Tm/A
d) 3 × 10^8 m/s
The electrostatic force is a:
a) Vector quantity
b) Scalar quantity
c) Either vector or scalar depending on context
d) None of the above
Coulomb’s law applies to:
a) Only charged conductors
b) Only charged insulators
c) Any charged particles
d) Only magnetic charges
If the charges are tripled, the force between them:
a) Remains unchanged
b) Triples
c) Becomes nine times greater
d) Becomes one-third
In Coulomb’s law, the force is inversely proportional to:
a) The square of the product of the charges
b) The distance between the charges
c) The sum of the charges
d) The square of the distance between the charges
The electrostatic force between two point charges in a vacuum is:
a) Independent of the charges
b) Dependent on the medium
c) Directly proportional to the product of the charges
d) Inversely proportional to the square of the charges
The electrostatic force between two charges depends on:
a) The speed of the charges
b) The magnetic field between them
c) The magnitude of the charges and the distance between them
d) The gravitational force between them
If the distance between two charges is tripled, the force between them:
a) Becomes one-ninth
b) Becomes one-third
c) Doubles
d) Quadruples
The electrostatic force between two charges is directly proportional to:
a) The charge of one particle
b) The square of the distance between the charges
c) The sum of the charges
d) The product of the charges
The direction of the electrostatic force between two charges is:
a) Along the line connecting the charges
b) Perpendicular to the line connecting the charges
c) Opposite to the direction of gravity
d) Randomly oriented
If one charge is positive and the other is negative, the electrostatic force between them is:
a) Repulsive
b) Attractive
c) Zero
d) Indeterminate
The unit of permittivity is:
a) Farad per meter (F/m)
b) Newton per Coulomb (N/C)
c) Joule per Coulomb (J/C)
d) Volt per meter (V/m)
In Coulomb’s law, the distance between the charges is measured in:
a) Meters
b) Centimeters
c) Millimeters
d) Kilometers
The electrostatic force between two identical charges is:
a) Independent of the medium
b) Always attractive
c) Always repulsive
d) Zero
Coulomb’s law is most accurate for:
a) Charges placed in a high magnetic field
b) Charges at very high speeds
c) Point charges or spherically symmetric charges
d) Charges in a turbulent medium
If two charges are placed in a medium with dielectric constant εr = 2, the force between them is:
a) Halved
b) Doubled
c) Same as in vacuum
d) Quadrupled
The Coulomb constant k can also be expressed as:
a) 1 / (4π ε0)
b) 4π ε0
c) 1 / (2π ε0)
d) 2π ε0
The force between two charges of opposite sign is:
a) Always attractive
b) Always repulsive
c) Zero
d) Indeterminate
In Coulomb’s law, ε0 represents:
a) The permittivity of free space
b) The magnetic permeability of free space
c) The dielectric constant of the medium
d) The gravitational constant
The force between two charges separated by a distance of 0.5 meters is compared to the force at 1 meter. It will be:
a) Four times greater
b) Half as great
c) Twice as great
d) One-fourth as great
The concept of Coulomb’s law is named after:
a) Isaac Newton
b) Michael Faraday
c) Charles-Augustin de Coulomb
d) James Clerk Maxwell
If you are interested to enhance your knowledge regarding Physics, Chemistry, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.