Factors Affecting Rate Of Reaction MDCAT Quiz with Answers
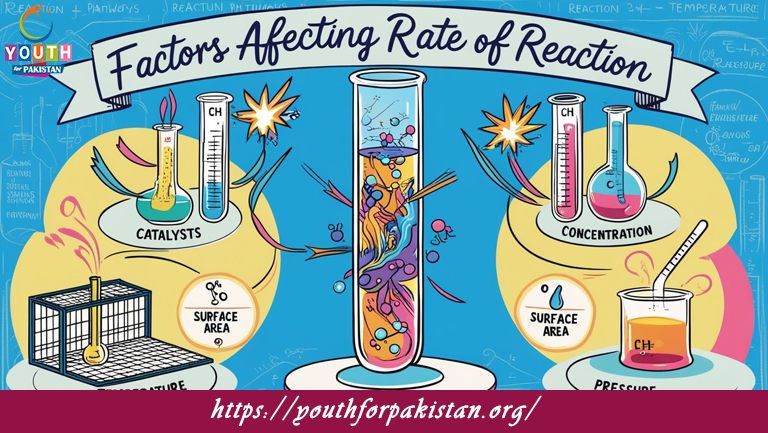
Factors Affecting Rate Of Reaction MDCAT Quiz: how various conditions affect the rate of chemical reactions. The rate of a chemical reaction is determined by factors such as the reactant concentration, temperature, surface area, and the presence of catalysts. These are the parameters to master in solving MDCAT Quiz questions relating to reaction kinetics and understanding how to manipulate these variables to control reaction rates.
Concentration and Rate of Reaction
The concentration of reactants directly affects the rate of reaction. In essence, increased reactant concentration means more particles will be available for collision; hence, increasing the chances of successful collisions. This increases the rate of reaction. For MDCAT students, understanding how to apply this in practice to different reactions is very important because you may be expected to predict how an increase or decrease in the concentration will alter the rate of a given reaction in MDCAT Quiz questions.
Temperature, Surface Area, and Catalysts
Temperature is another factor that affects the rate of reaction. Higher temperatures provide reactant molecules with more energy, making collisions more frequent and effective. As a result, increasing the temperature typically increases the reaction rate. Similarly, increasing the surface area of solid reactants exposes more particles to react, which speeds up the reaction. The presence of a catalyst also significantly influences reaction rates by lowering the activation energy needed for the reaction to occur. Understanding these factors and how they influence the rate of reaction is essential for MDCAT students. Free Flashcard resources can be useful in memorizing the different effects of these factors on reaction rates.
By mastering the factors affecting the rate of reaction, MDCAT students can perform well in both theoretical and experimental sections of the exam. Using MDCAT Quiz and Free Flashcard tools will help reinforce key concepts, improve problem-solving skills, and boost your confidence for tackling questions related to reaction kinetics.

The increase in the concentration of _______ generally increases the rate of enzyme activity.
Substrate

The amount of _______ can affect the rate of enzyme action by either inhibiting or activating enzymes.
Cofactors

At high substrate concentrations, the enzyme reaches _______ where increasing substrate does not affect the rate.
Saturation

An enzyme will stop working at a _______ temperature due to the denaturation of its active site.
High

_______ inhibitors bind to a site other than the active site and alter the enzyme's structure.
Non-competitive

Enzyme activity decreases when the substrate concentration is _______ relative to enzyme concentration.
High

If a substrate concentration exceeds the _______ of the enzyme, the rate of reaction will plateau.
Binding capacity

At low substrate concentrations, the rate of enzyme activity is _______ to the substrate amount.
Directly proportional

Enzyme activity can be modulated by the presence of _______ molecules that stabilize their active form.
Activators

Enzymes are _______ when they lose their three-dimensional structure due to environmental factors.
Denatured

The rate of enzyme activity is affected by the _______ of the enzyme in the reaction mixture.
Concentration

The concentration of _______ is typically kept constant in the cell, but its availability affects enzyme action.
Coenzymes
Experience the real exam environment with our expertly designed collection of over 25,000 MCQs MDCAT Mock Tests.