Explanation Of Electrolysis MDCAT Quiz with Answers
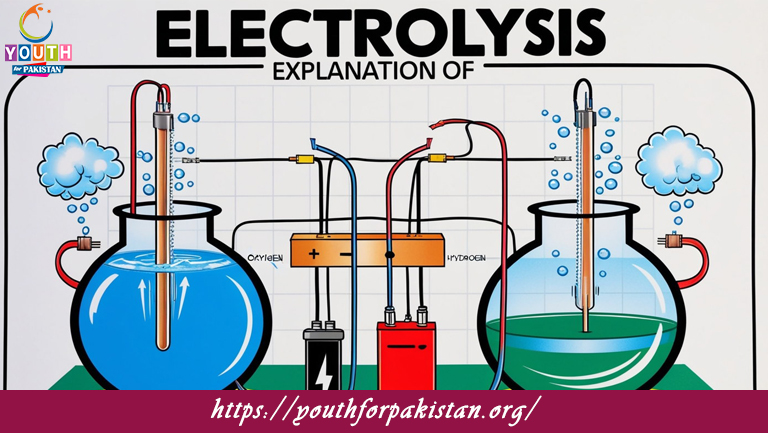
Explanation Of Electrolysis MDCAT Quiz: process of breaking down compounds using electricity. Electrolysis is used to extract metals, refine chemicals, and perform other essential reactions. For MDCAT preparation, understanding the fundamentals of electrolysis, including the role of electrodes, electrolyte solutions, and the flow of electric current, is key to answering related questions in the MDCAT Quiz. Mastering this concept is vital for both theoretical understanding and practical applications in the field of chemistry.
H2: The Process of Electrolysis
Electrolysis involves the passage of an electric current through an electrolyte, which is a substance that conducts electricity because it contains ions. As the electric current passes, the positive ions move toward the negative electrode, which is called the cathode, and the negative ions move toward the positive electrode, which is called the anode. When the ions reach the electrodes, oxidation and reduction reactions occur. These reactions lead to the decomposition of the compound into its constituent elements. The products of electrolysis depend on the nature of the electrolyte and conditions like voltage and concentration. Understanding these principles is essential for solving questions on electrolysis in MDCAT.
H3: Quiz on Electrolysis
The MDCAT Quiz on Electrolysis helps to test whether students are familiar with the major concepts and the reactions that happen during electrolysis. Students are expected to identify products of electrolysis, describe what happens at electrodes, and learn how to calculate amounts of substances produced during electrolysis. These quizzes offer practice in many types of electrolysis, such as water, salts, and molten compounds. By doing the quiz, students can revisit their concepts on electrolysis and move into the MDCAT exam with a much more confident attitude.
H3: Free Flashcard for Electrolysis
To help them further in preparation, MDCAT students may use the Free Flashcard for electrolysis. Such flashcards will provide a quick summary of key terms like anode, cathode, electrolyte, and electrolysis products. Review of the flashcards aids relearning and thus enables students to answer questions reviewed during revision quickly. The flashcards make a very good tool for better retention and effective mastering of electrolysis so as to face any kind of question related to the subject on the MDCAT exam.
Experience the real exam environment with our expertly designed collection of over 25,000 MCQs MDCAT Mock Tests.






