Half Life And Rate Of Decay MDCAT Quiz with Answers
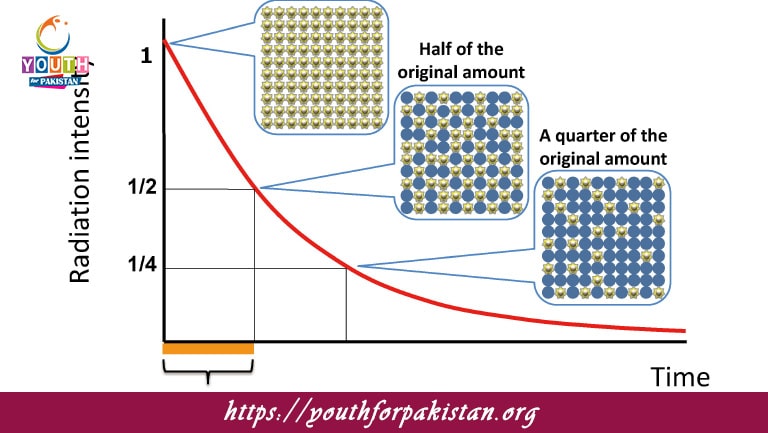
Half Life And Rate Of Decay MDCAT Quiz, the time required by half of the atoms of a sample containing a radioactive substance to decay is called half-life. This concept is an essential part of nuclear physics and describes radioactive decay in a predictable manner. The rate of decay is determined by probabilities that a single atom undergoes decay in a given time frame, which remains constant for a given radioactive isotope. The process of decay in an exponential mode shows a decrease in the quantity of undecayed nuclei by half on every consecutive half-life.
Test Your Knowledge with an MDCAT Quiz
The MDCAT Quiz on Half-Life and Rate of Decay is designed to help you check your skills in computing the amount of the remaining radioactive substance after a certain time or calculating half-life given the decay constant. The quizzes cover such topics as activity calculation, the relationship between half-life and decay constant, and just how the rate of decay affects the stability of radioactive materials. Regular practice will prepare you to solve these problems efficiently in the MDCAT exam.
- Test Name: Half Life And Rate Of Decay MDCAT Quiz
- Type: Quiz Test
- Total Questions: 30
- Total Marks: 30
- Time: 30 minutes
Note: Answer of the questions will change randomly each time you start the test, once you are finished, click the View Results button.
Free Flashcards for Quick Revision
Free Flashcards on Half-Life and Rate of Decay provide quick access to important formulas, including the calculation of half-life, decay constant, and activity of a radioactive sample. These flashcards are an excellent revision tool for rapidly recalling key concepts and formulas that will help you solve questions related to nuclear decay in the MDCAT exam.

The rate of decay of a radioactive substance is directly proportional to:
The number of undecayed nuclei

If the half-life of a substance is 5 years, after 10 years, the fraction of the original sample remaining will be:
45661

If a radioactive substance has a half-life of 10 years, then after 20 years, what fraction of the original substance will remain?
45661

If the half-life of a substance is 4 hours, after 12 hours, what fraction of the original sample remains?
45665
Experience the real exam environment with our expertly designed collection of over 25,000 MCQs MDCAT Mock Tests.