Spontaneous And Random Nuclear Decay MDCAT Quiz
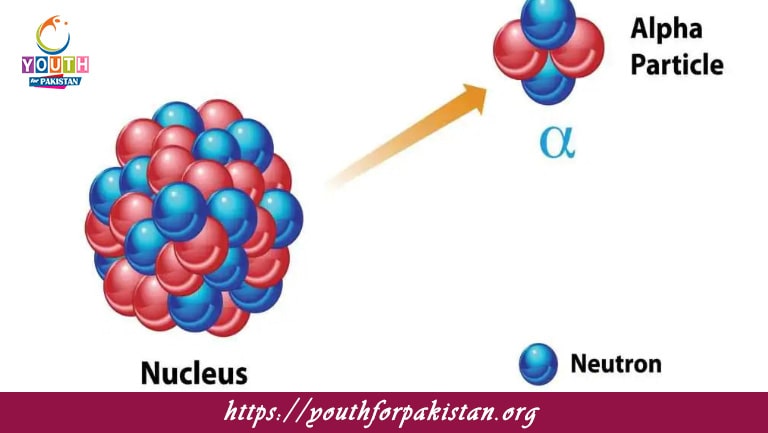
Spontaneous And Random Nuclear Decay MDCAT Quiz refers to the process in which an unstable atomic nucleus loses energy by emitting radiation, such as alpha particles, beta particles, or gamma rays, without any external influence. This decay is spontaneous, meaning it occurs naturally due to the instability of the nucleus, which may be caused by an excess of energy or an imbalance between protons and neutrons. The decay process is random, and while the probability of decay per unit time is constant for each radioactive isotope, it is impossible to predict the exact moment any individual nucleus will decay. The rate of decay for a large collection of nuclei is typically characterized by the half-life, which is the time it takes for half of the nuclei in a sample to undergo decay. For MDCAT students, understanding the principles behind spontaneous and random nuclear decay is crucial for solving problems related to radioactive decay, half-life, and nuclear reactions.
Test Your Knowledge with an MDCAT Quiz
An MDCAT Quiz on Spontaneous and Random Nuclear Decay would help one reinforce the concepts regarding the behavior of radioactive substances. It covers key subjects such as how to calculate the decay constant and determine the half-life of substances, and types of radioactive decay, including alpha, beta, and gamma. Regular exposure to these forms of quizzes by practicing will eventually prepare you to answer questions set forth on MDCAT in relation to its probabilistic nature of radioactive decay and mathematical tools necessary for its description.
- Test Name: Spontaneous And Random Nuclear Decay MDCAT Quiz
- Type: Quiz Test
- Total Questions: 30
- Total Marks: 30
- Time: 30 minutes
Note: Answer of the questions will change randomly each time you start the test, once you are finished, click the View Results button.
Free Flashcards for Quick Revision
Free Flashcards on Spontaneous and Random Nuclear Decay: Quick, concise summaries of key concepts including half-life, types of radiation, and the decay law. These flashcards will help you rapidly recall important formulas, definitions, and principles of radioactive decay—perfect for last-minute revision before your MDCAT exam.
Experience the real exam environment with our expertly designed collection of over 25,000 MCQs MDCAT Mock Tests.