Specific Heat Capacity MDCAT Quiz with Answers
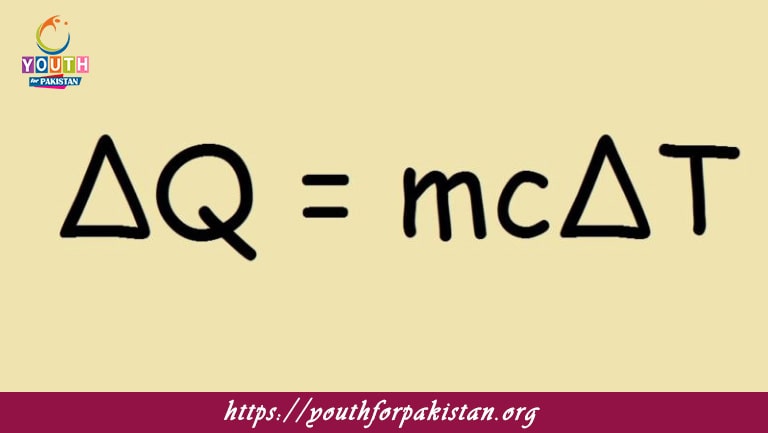
Specific Heat Capacity refers to the amount of heat energy needed to raise the temperature of a unit mass of any substance by one degree Celsius, or Kelvin. The concept becomes essentially important in thermodynamics, since it explains how various materials respond to heat. Most MDCAT students need an understanding of the topic of specific heat capacity in order to solve problems associated with heat transfer, energy conservation, and temperature changes.
Definition and Formula
Specific heat capacity (
????
c) is mathematically expressed as:
????
=
????
????
Δ
????
c=
mΔT
Q
Here:
????
c is the specific heat capacity (J/kg·K),
????
Q is the heat energy absorbed or released (Joules),
????
m is the mass of the substance (kg),
Δ
????
ΔT is the change in temperature (°C or K).
The higher the specific heat capacity of a substance, the more heat energy it requires to change its temperature. For instance, water has a high specific heat capacity, hence it’s very good at moderating temperature within the environment and living organisms.
Importance of Specific Heat Capacity
Heat Storage and Transfer: Materials possessing high specific heat capacities, such as water, can absorb extensive quantities of heat energy with small changes in temperature. This characteristic is utilized in climate regulation, cooking, and thermal storage systems.
Practical Applications:
Engineering: Specific heat is crucial in designing cooling and heating systems, such as radiators and heat exchangers.
Medicine: It explains how the human body maintains temperature by means such as sweating.
Astronomy: Helps in understanding planetary heat dynamics and atmospheric behavior.
Role in Phase Transitions: During phase changes, such as melting or boiling, the heat energy is used to overcome intermolecular forces rather than changing temperature, which is explained using specific heat capacity.
MDCAT Quiz: Specific Heat Capacity
The MDCAT Quiz on Specific Heat Capacity assesses students’ understanding of heat transfer, energy calculations, and real-world applications. Questions may involve using the formula
????
=
????
????
Δ
????
Q=mcΔT, solving the unknowns in a problem or the interpretation of experimental data—makes students comfortable when dealing with higher-level issues of thermodynamics.
Free Flashcards for Specific Heat Capacity
Free flashcards for specific heat capacity are a very good way to reinforce this concept. The flashcards may include important formulas, units, example problems, and comparative values of specific heat capacities of common substances. Regular practice with these flashcards will surely help the student strengthen their concepts and problem-solving skills, ensuring success in MDCAT physics topics.