Stoichiometry Quiz with Answers

Welcome to the Stoichiometry MCQs with Answers, it helps learners quickly identify areas for improvement in Stoichiometry Online Test.
| Stoichiometry is a crucial aspect of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It relies heavily on the concept of balanced chemical equations, which ensure that the number of atoms for each element is conserved during the reaction. To excel in stoichiometry, students often practice with Stoichiometry MCQs with Answers, which help in understanding and applying these relationships.
Balanced Chemical Equations MCQs with Answers are essential for mastering the skill of balancing equations, a fundamental step in any stoichiometric calculation. Once equations are balanced, students can use Mole Ratio MCQs with Answers to practice converting between moles of reactants and products based on these ratios. Mole-to-Mole Conversions MCQs with Answers focus on these conversions, ensuring that students can accurately determine the amount of one substance needed or produced given a certain amount of another. Similarly, Mass-to-Mass Conversions MCQs with Answers help students practice converting between the mass of reactants and products, using molar masses and balanced equations. One of the more challenging concepts in stoichiometry is identifying the limiting reactant, the substance that is completely consumed first, limiting the amount of product formed. Limiting Reactant MCQs with Answers provide valuable practice in determining which reactant will limit the reaction and calculating the theoretical yield based on this reactant. These MCQs not only reinforce theoretical understanding but also prepare students for practical applications in laboratory settings, enhancing their problem-solving skills and conceptual clarity in stoichiometry. |
Stoichiometry Online Quiz
By presenting 3 options to choose from, Stoichiometry Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Stoichiometry Questions and Answers and click over the correct answer.
- Test Name: Stoichiometry MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Stoichiometry Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Stoichiometry Flashcards

What is the branch of chemistry that deals with the calculation of quantities of substances involved in chemical reactions?

The ratio of the number of moles of each substance in a chemical equation is determined by the ______.

What is the process of determining the amounts of reactants and products in a chemical reaction called?

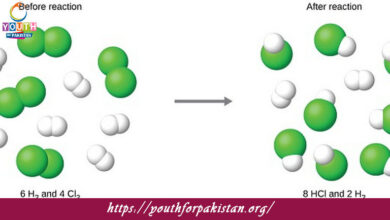
How many moles of hydrogen gas are required to react completely with 1 mole of oxygen gas to produce water according to the balanced chemical equation 2H₂ + O₂ → 2H₂O?

The stoichiometric coefficient of a reactant or product in a balanced chemical equation represents the ______.

How many moles of oxygen gas are required to produce 3 moles of water according to the balanced chemical equation 4H₂ + 2O₂ → 4H₂O?

The stoichiometric ratio of reactants and products in a chemical equation is determined by the ______.

How many grams of water are produced when 4 moles of hydrogen gas react completely with excess oxygen gas according to the balanced chemical equation 2H₂ + O₂ → 2H₂O?

How many moles of carbon dioxide (CO₂) are produced when 2 moles of methane gas (CH₄) react completely with excess oxygen gas according to the balanced chemical equation CH₄ + 2O₂ → CO₂ + 2H₂O?

What is the stoichiometric coefficient of oxygen gas (O₂) in the balanced chemical equation for the combustion of propane (C₃H₈) to produce carbon dioxide and water?

How many grams of oxygen gas are required to react completely with 5 grams of hydrogen gas to produce water according to the balanced chemical equation 2H₂ + O₂ → 2H₂O? (Assume excess oxygen.)

What is the stoichiometric coefficient of carbon dioxide (CO₂) in the balanced chemical equation for the combustion of methane (CH₄) to produce carbon dioxide and water?

How many moles of methane (CH₄) are needed to produce 4 moles of water according to the balanced chemical equation CH₄ + 2O₂ → CO₂ + 2H₂O?

How many grams of ammonia (NH₃) are produced when 4 moles of nitrogen gas react completely with excess hydrogen gas according to the balanced chemical equation N₂ + 3H₂ → 2NH₃?

What is the stoichiometric coefficient of hydrogen gas (H₂) in the balanced chemical equation for the combustion of propane (C₃H₈) to produce carbon dioxide and water?

How many grams of oxygen gas are required to react completely with 10 grams of propane (C₃H₈) to produce carbon dioxide and water according to the balanced chemical equation C₃H₈ + 5O₂ → 3CO₂ + 4H₂O?

What is the stoichiometric coefficient of water (H₂O) in the balanced chemical equation for the combustion of propane (C₃H₈) to produce carbon dioxide and water?

How many moles of oxygen gas are required to produce 2 moles of carbon dioxide according to the balanced chemical equation C₃H₈ + 5O₂ → 3CO₂ + 4H₂O?

How many grams of carbon dioxide (CO₂) are produced when 5 moles of propane (C₃H₈) react completely with excess oxygen gas according to the balanced chemical equation C₃H₈ + 5O₂ → 3CO₂ + 4H₂O?

What is the stoichiometric coefficient of propane (C₃H₈) in the balanced chemical equation for its combustion to produce carbon dioxide and water?

How many moles of carbon dioxide (CO₂) are produced when 3 moles of ethane (C₂H₆) react completely with excess oxygen gas according to the balanced chemical equation 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O?

How many grams of water are produced when 2 moles of ethane (C₂H₆) react completely with excess oxygen gas according to the balanced chemical equation 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O?

What is the stoichiometric coefficient of water (H₂O) in the balanced chemical equation for the combustion of ethane (C₂H₆) to produce carbon dioxide and water?

How many grams of oxygen gas are required to react completely with 3 grams of ethane (C₂H₆) to produce carbon dioxide and water according to the balanced chemical equation 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O?

What is the stoichiometric coefficient of ethane (C₂H₆) in the balanced chemical equation for its combustion to produce carbon dioxide and water?

How many moles of carbon dioxide (CO₂) are produced when 4 moles of ethane (C₂H₆) react completely with excess oxygen gas according to the balanced chemical equation 2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O?

How many moles of oxygen gas are required to produce 2 moles of water according to the balanced chemical equation 2H₂ + O₂ → 2H₂O?
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.