Welcome to the Redox Reactions MCQs with Answers, it helps learners quickly identify areas for improvement in Redox Reactions Online Test.
| Redox (reduction-oxidation) reactions involve the transfer of electrons between reactants, where one species undergoes oxidation (loses electrons) and another undergoes reduction (gains electrons). These reactions are fundamental in chemistry, occurring in processes ranging from energy production to biological functions.
In a redox reactions quiz, MCQs on redox reactions typically cover essential concepts and applications. Oxidation-reduction multiple choice questions explore the identification of oxidizing and reducing agents, understanding oxidation states, and predicting reaction spontaneity based on standard reduction potentials.
Balancing redox equations MCQs focus on techniques such as the half-reaction method and the oxidation number method for balancing chemical equations involving redox reactions, ensuring conservation of mass and charge.
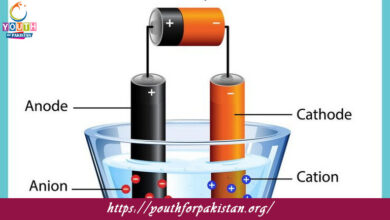
Electrochemical cells exam questions delve into the principles and applications of redox reactions in electrochemical cells, including galvanic cells (voltaic cells) and electrolytic cells, and their role in generating electricity or driving non-spontaneous reactions.
Half-reactions MCQs examine the individual oxidation and reduction processes within redox reactions, emphasizing their role in understanding reaction mechanisms and calculating electrode potentials. |
Redox Reactions Online Quiz
By presenting 3 options to choose from, Redox Reactions Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Redox Reactions Questions and Answers and click over the correct answer.
- Test Name: Redox Reactions MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Redox Reactions Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Redox Reactions Flashcards

What is the process in which one substance loses electrons while another gains them?

In a redox reaction, the species that gains electrons is said to be _______.

Which of the following is NOT a characteristic of oxidation?

Reduction is the _______.

What is the reducing agent in a redox reaction?
The substance that undergoes oxidation

The oxidation state of an atom in an element is _______.

What happens to the oxidation state of an element when it is oxidized?

Which of the following statements about redox reactions is true?
The total number of electrons lost must equal the total number gained

What is the oxidation state of oxygen in most compounds?

What is the oxidation state of hydrogen in most compounds?

Which of the following elements has a variable oxidation state?

Which of the following is a disproportionation reaction?
A redox reaction where the same element is both oxidized and reduced

What is the oxidation state of chlorine in HCl?

The oxidation state of an atom in its elemental form is _______.

In the reaction 2H2O2 → 2H2O + O2, hydrogen peroxide acts as a _______.

What is the oxidation state of carbon in methane (CH4)?

What is the reducing agent in the reaction 2Fe2O3 + 3C → 4Fe + 3CO2?

Which of the following elements has the highest oxidation state?

What is the oxidation state of sulfur in H2SO4?

What is the reducing agent in the reaction 2KMnO4 + 5H2C2O4 + 3H2SO4 → 2MnSO4 + 10CO2 + 8H2O?

Which of the following statements about oxidation is true?
It involves a decrease in the oxidation state

In the reaction Zn + 2HCl → ZnCl2 + H2, zinc is _______.

What is the oxidation state of nitrogen in NH3?

What is the oxidizing agent in the reaction 2KBr + Cl2 → 2KCl + Br2?

What is the oxidation state of manganese in KMnO4?

Which of the following statements about redox reactions is false?
The substance that gains electrons is oxidized

What is the oxidation state of phosphorus in PH3?

In the reaction 2Na + Cl2 → 2NaCl, sodium is _______.

What is the reducing agent in the reaction 2H2O2 → 2H2O + O2?

Which of the following statements about redox reactions is true?
The substance that loses electrons is oxidized

What is the oxidation state of oxygen in H2O2?

What is the oxidizing agent in the reaction Zn + CuSO4 → ZnSO4 + Cu?

Which of the following elements can have a positive or negative oxidation state?

What is the oxidation state of potassium in K2SO4?

What is the reducing agent in the reaction 2KClO3 → 2KCl + 3O2?

In the reaction 2Mg + O2 → 2MgO, magnesium is _______.

What is the oxidation state of chlorine in Cl2?

Which of the following elements is the most common reducing agent in organic chemistry?

What is the oxidation state of sulfur in SO2?

What is the oxidizing agent in the reaction 2Fe + 3Cl2 → 2FeCl3?
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.