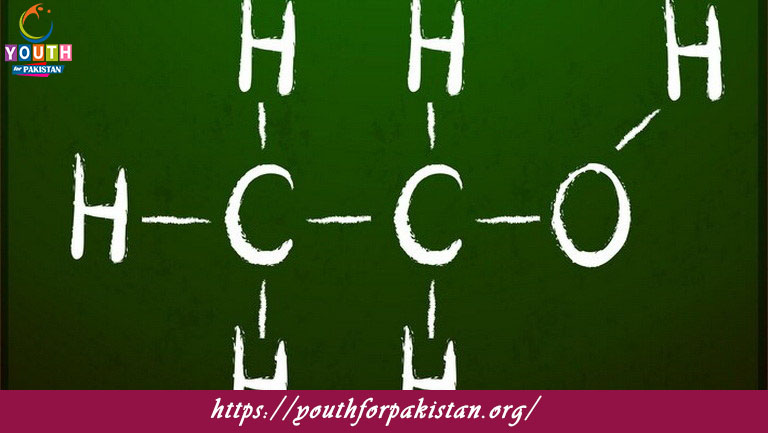
Welcome to the Reaction Mechanisms MCQs with Answers, it helps learners quickly identify areas for improvement in Reaction Mechanisms Online Test.
| Reaction mechanisms are crucial in understanding how chemical reactions occur at a molecular level. They detail the step-by-step process by which reactants transform into products, including the formation of intermediates. Studying reaction mechanisms is fundamental in organic chemistry, as it provides insights into the behavior and reactivity of various compounds.
For those preparing for exams or assessments, Reaction Mechanisms MCQs are an excellent way to test comprehension. Organic Chemistry Reaction Mechanisms MCQs focus specifically on the mechanisms prevalent in organic reactions, such as nucleophilic substitutions, electrophilic additions, and free radical reactions. These multiple choice questions help in identifying key steps and intermediates involved in reactions.
Chemical Reaction Mechanisms Multiple Choice Questions often include topics like transition states, reaction coordinates, and energy profiles. These questions are designed to challenge and enhance understanding of complex reaction pathways. Taking Reaction Mechanisms Quiz Questions can significantly aid in mastering the subject, as they cover a broad spectrum of mechanisms and their intermediates.
Advanced Reaction Mechanisms Questions delve deeper into sophisticated topics, requiring a more profound knowledge of the principles and applications of reaction mechanisms. MCQs on Reaction Mechanisms and Intermediates test the ability to predict products, understand the kinetics of reactions, and recognize various reaction intermediates, ensuring a comprehensive grasp of the subject. |
Reaction Mechanisms Online Quiz
By presenting 3 options to choose from, Reaction Mechanisms Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Reaction Mechanisms Questions and Answers and click over the correct answer.
- Test Name: Reaction Mechanisms MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Reaction Mechanisms Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Reaction Mechanisms Flashcards

What is the slowest step in a reaction mechanism called?

Which type of intermediate is commonly involved in radical chain reactions?

What is a reaction mechanism?
Step-by-step sequence of elementary reactions

Which step involves the formation of a carbocation?

What is the term for the series of steps that describe the pathway from reactants to products?

In an SN2 reaction, what is the molecularity of the rate-determining step?

In the reaction mechanism for an SN1 reaction, what is the first step?
Formation of a carbocation

What type of reaction mechanism involves a single step?

Which term describes a substance that increases the rate of a reaction without being consumed?

In a catalytic reaction, what is typically the role of the catalyst?

What is the intermediate called in an E1 reaction mechanism?

What type of mechanism involves radicals?

Which step in a radical chain reaction terminates the reaction?

What is the transition state in a reaction mechanism?

Which mechanism involves the simultaneous bond breaking and bond forming?

In an SN1 reaction, what is the rate-determining step dependent on?
Concentration of the substrate

Which step in an SN2 reaction mechanism is the slowest?

What is a reaction intermediate?
Species formed during the reaction that does not appear in the overall equation

In an E2 elimination reaction, how many molecules are involved in the transition state?

Which type of reaction mechanism involves a two-step process?

What happens during the propagation step of a radical chain reaction?
Radicals react and form new radicals

In a multi-step reaction, what determines the overall reaction rate?

What is a termolecular reaction?
Reaction involving three molecules

In an SN2 mechanism, what happens to the stereochemistry of the substrate?

What role does the solvent play in an SN1 reaction mechanism?
Stabilizes the carbocation

What is the term for the energy difference between reactants and the transition state?

In an E1 reaction, what is the first step?
Formation of a carbocation

In a chain reaction, what is the role of the initiation step?
Generates reactive intermediates

What is a concerted mechanism?
A mechanism where bond breaking and forming occur simultaneously

Which intermediate is common in the mechanism of acid-catalyzed hydration of alkenes?

In a reaction mechanism, what is a transition state?
High-energy, unstable state

In an E2 mechanism, what is the orientation of the leaving groups?

Which step in a radical chain reaction produces the most products?

What is the result of the rate-determining step in a multi-step reaction?

In an SN1 reaction, what happens to the stereochemistry of the substrate?

Which type of mechanism involves intermediates?

What is the effect of a catalyst on the activation energy of a reaction?

In an E1 elimination mechanism, how many steps are involved?

What is the primary factor determining the rate of an SN2 reaction?
Concentration of both nucleophile and substrate

In a reaction mechanism, what role does the transition state play?
Represents the highest energy point
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.