Percent Yield MCQs with Answers

Welcome to the Percent Yield MCQs with Answers, it helps learners quickly identify areas for improvement in Percent Yield Online Test.
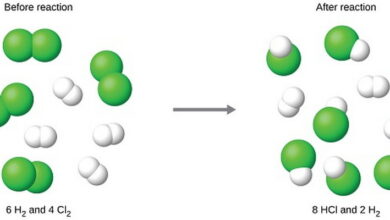
| Percent yield is a measure of the efficiency of a chemical reaction, indicating the percentage of the theoretical yield that is actually obtained in a practical or laboratory setting. It is calculated by dividing the actual yield (the amount of product obtained experimentally) by the theoretical yield (the maximum amount of product that can be obtained based on stoichiometric calculations) and multiplying by 100.
In a percent yield quiz, MCQs on percent yield typically assess understanding of how to calculate percent yield, interpret reaction efficiency, and identify factors that affect yield. Percent yield multiple choice questions often cover topics such as sources of experimental error, ways to improve yield, and the implications of low or high percent yields in chemical processes. Reaction efficiency MCQs focus on the relationship between percent yield and reaction stoichiometry, exploring strategies to maximize product formation and minimize waste. Yield calculation exam questions challenge students to apply their knowledge of percent yield to analyze and interpret experimental data from chemical reactions. Chemical reactions percent yield MCQs delve into scenarios where students must calculate percent yield for different types of reactions, ranging from simple to complex reaction pathways. |
Percent Yield Online Quiz
By presenting 3 options to choose from, Percent Yield Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Percent Yield Questions and Answers and click over the correct answer.
- Test Name: Percent Yield MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Percent Yield Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Percent Yield Flashcards
If the theoretical yield of a reaction is 50 grams and the actual yield is 45 grams, what is the percent yield?
0.9
If a reaction has a theoretical yield of 120 grams and an actual yield of 96 grams, what is the percent yield?
0.8
A reaction has a theoretical yield of 200 grams but only produces 150 grams. What is the percent yield?
0.75
If the theoretical yield of a product is 300 grams and the actual yield is 270 grams, what is the percent yield?
0.9
If you obtain 40 grams of a product from a reaction with a theoretical yield of 50 grams, what is the percent yield?
0.8
A reaction with a theoretical yield of 400 grams produces 360 grams of product. What is the percent yield?
0.9
The actual yield of a product is 25 grams, and the theoretical yield is 50 grams. What is the percent yield?
0.5
If the actual yield of a reaction is 200 grams and the theoretical yield is 250 grams, what is the percent yield?
0.8
A reaction has a theoretical yield of 100 grams and an actual yield of 70 grams. What is the percent yield?
0.7
If you recover 48 grams of a product from a reaction with a theoretical yield of 60 grams, what is the percent yield?
0.8
The theoretical yield of a reaction is 500 grams, and the actual yield is 400 grams. What is the percent yield?
0.8
If a reaction with a theoretical yield of 350 grams produces 315 grams of product, what is the percent yield?
0.9
A reaction has a theoretical yield of 250 grams but only produces 200 grams. What is the percent yield?
0.8
If the actual yield of a reaction is 72 grams and the theoretical yield is 90 grams, what is the percent yield?
0.8
The theoretical yield of a product is 150 grams, and the actual yield is 120 grams. What is the percent yield?
0.8
If you obtain 36 grams of a product from a reaction with a theoretical yield of 45 grams, what is the percent yield?
0.8
A reaction with a theoretical yield of 600 grams produces 540 grams of product. What is the percent yield?
0.9
The actual yield of a product is 30 grams, and the theoretical yield is 60 grams. What is the percent yield?
0.5
If the actual yield of a reaction is 400 grams and the theoretical yield is 500 grams, what is the percent yield?
0.8
A reaction has a theoretical yield of 80 grams and an actual yield of 64 grams. What is the percent yield?
0.8
If you recover 45 grams of a product from a reaction with a theoretical yield of 50 grams, what is the percent yield?
0.9
The theoretical yield of a reaction is 300 grams, and the actual yield is 240 grams. What is the percent yield?
0.8
If a reaction with a theoretical yield of 150 grams produces 120 grams of product, what is the percent yield?
0.8
A reaction has a theoretical yield of 500 grams but only produces 450 grams. What is the percent yield?
0.9
If the actual yield of a reaction is 90 grams and the theoretical yield is 100 grams, what is the percent yield?
0.9
The theoretical yield of a product is 250 grams, and the actual yield is 200 grams. What is the percent yield?
0.8
If you obtain 54 grams of a product from a reaction with a theoretical yield of 60 grams, what is the percent yield?
0.9
A reaction with a theoretical yield of 400 grams produces 320 grams of product. What is the percent yield?
0.8
The actual yield of a product is 50 grams, and the theoretical yield is 100 grams. What is the percent yield?
0.5
If the actual yield of a reaction is 300 grams and the theoretical yield is 400 grams, what is the percent yield?
0.75
A reaction has a theoretical yield of 200 grams and an actual yield of 150 grams. What is the percent yield?
0.75
If you recover 90 grams of a product from a reaction with a theoretical yield of 100 grams, what is the percent yield?
0.9
The theoretical yield of a reaction is 500 grams, and the actual yield is 400 grams. What is the percent yield?
0.8
If a reaction with a theoretical yield of 600 grams produces 480 grams of product, what is the percent yield?
0.8
A reaction has a theoretical yield of 100 grams but only produces 80 grams. What is the percent yield?
0.8
If the actual yield of a reaction is 50 grams and the theoretical yield is 60 grams, what is the percent yield?
0.8333
The theoretical yield of a product is 350 grams, and the actual yield is 280 grams. What is the percent yield?
0.8
If you obtain 90 grams of a product from a reaction with a theoretical yield of 120 grams, what is the percent yield?
0.75
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.