Enthalpy MCQs with Answers
Welcome to the Enthalpy MCQs with Answers, it helps learners quickly identify areas for improvement in Enthalpy Online Test.
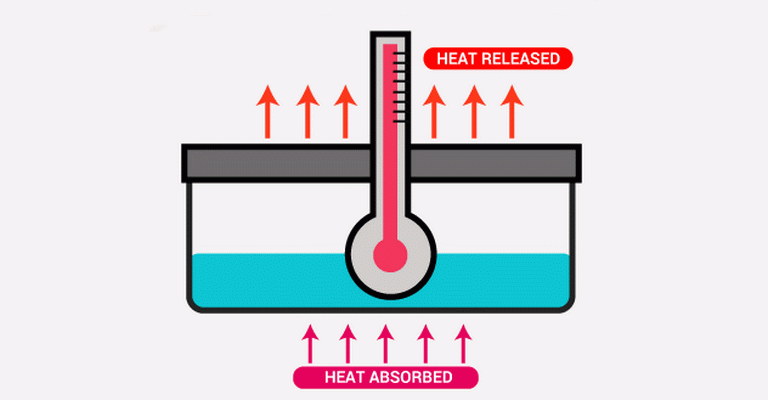
| Enthalpy is a fundamental concept in thermodynamics that represents the total heat content of a system at constant pressure. It includes the internal energy of the system plus the product of pressure and volume. Enthalpy is crucial for understanding and quantifying heat transfer in various processes, including chemical reactions, phase changes, and physical transformations.
In an enthalpy quiz, MCQs on enthalpy typically assess understanding of its definition and calculation, often focusing on ΔH (change in enthalpy) values for different reactions and scenarios. Enthalpy multiple choice questions delve into topics such as heat capacity, enthalpy of formation, and enthalpy of reaction, testing knowledge of how these quantities relate to energy changes in chemical systems. Heat and enthalpy MCQs explore the principles of calorimetry and heat transfer, including how to measure and interpret enthalpy changes experimentally. Enthalpy change exam questions challenge students to apply enthalpy concepts to analyze energy flow and transformation in practical situations, such as determining the heat released or absorbed during a reaction. Thermochemistry enthalpy MCQs cover broader applications of enthalpy in thermochemical equations and Hess’s law, emphasizing its role in predicting and interpreting energy changes in chemical reactions. |
Enthalpy Online Quiz
By presenting 3 options to choose from, Enthalpy Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Enthalpy Questions and Answers and click over the correct answer.
- Test Name: Enthalpy MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Enthalpy Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Enthalpy Flashcards
What is the equation for calculating the change in enthalpy (ΔH) of a reaction?
ΔH = H(products) - H(reactants)
Which enthalpy change is associated with the formation of one mole of a compound from its elements in their standard states?
Enthalpy of formation
What is the enthalpy change when a substance undergoes a phase transition at its melting point?
Enthalpy of fusion
What is the enthalpy change when a substance undergoes a phase transition at its boiling point?
Enthalpy of vaporization
What is the enthalpy change when a substance undergoes a phase transition at a temperature below its melting point?
Enthalpy of sublimation
What is the enthalpy change when a substance undergoes a phase transition at a temperature above its boiling point?
Enthalpy of sublimation
What is the enthalpy change when a substance undergoes a phase transition from solid to liquid?
Enthalpy of fusion
What is the enthalpy change when a substance undergoes a phase transition from liquid to solid?
-Enthalpy of fusion
What is the enthalpy change when a substance undergoes a phase transition from liquid to gas?
Enthalpy of vaporization
What is the enthalpy change when a substance undergoes a phase transition from gas to liquid?
-Enthalpy of vaporization
What is the enthalpy change when a substance undergoes a phase transition from gas to solid?
Enthalpy of sublimation
What is the enthalpy change when a substance undergoes a phase transition from solid to gas?
Enthalpy of sublimation
What is the enthalpy change when a substance undergoes a phase transition from gas to plasma?
Ionization energy
What is the enthalpy change when a substance undergoes a phase transition from plasma to gas?
-Ionization energy
What is the enthalpy change when a substance undergoes a phase transition from liquid to plasma?
Enthalpy of vaporization
What is the enthalpy change when a substance undergoes a phase transition from plasma to liquid?
-Enthalpy of vaporization
What is the enthalpy change when a substance undergoes a phase transition from solid to plasma?
Enthalpy of sublimation
What is the enthalpy change when a substance undergoes a phase transition from plasma to solid?
-Enthalpy of sublimation
What is the enthalpy change when a substance undergoes a phase transition from plasma to Bose-Einstein condensate?
Condensation energy
What is the enthalpy change when a substance undergoes a phase transition from Bose-Einstein condensate to plasma?
-Condensation energy
What is the enthalpy change when a substance undergoes a phase transition from liquid to Bose-Einstein condensate?
Condensation energy
What is the enthalpy change when a substance undergoes a phase transition from Bose-Einstein condensate to liquid?
-Condensation energy
What is the enthalpy change when a substance undergoes a phase transition from gas to Bose-Einstein condensate?
Condensation energy
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.