Corrosion MCQs with Answers

Welcome to the Corrosion MCQs with Answers, it helps learners quickly identify areas for improvement in Corrosion Online Test.
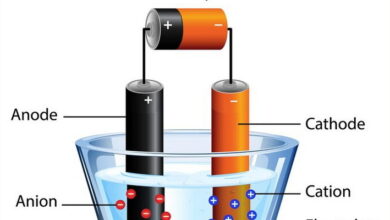
| Corrosion is a natural electrochemical process that occurs when metals react with their environment, leading to deterioration and loss of material integrity. This phenomenon is pervasive in industries such as construction, transportation, and manufacturing, impacting infrastructure, equipment, and safety.
In a corrosion quiz, MCQs on corrosion typically cover various aspects of this process. Types of corrosion multiple choice questions explore different forms such as uniform corrosion, pitting corrosion, galvanic corrosion, and crevice corrosion, highlighting their causes and effects on metals. Corrosion prevention methods MCQs focus on strategies to mitigate corrosion, including the use of protective coatings, inhibitors, alloying, cathodic protection, and design modifications. Rusting exam questions specifically address the oxidation of iron and steel in the presence of oxygen and moisture, a common form of corrosion. Corrosion mechanisms MCQs delve into the electrochemical reactions and environmental factors that accelerate metal degradation, including pH levels, temperature, and humidity. |
Corrosion Online Quiz
By presenting 3 options to choose from, Corrosion Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Corrosion Questions and Answers and click over the correct answer.
- Test Name: Corrosion MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Corrosion Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Corrosion Flashcards
What is the gradual destruction of metals by chemical or electrochemical reaction with their environment called?
Corrosion
What is the process of applying a protective zinc coating to iron or steel to prevent rusting called?
Galvanization
What is the chemical reaction responsible for the formation of rust on iron or steel?
Iron oxidation
What is the process of converting a metal surface into an oxide layer to protect it from corrosion called?
Passivation
Which of the following statements about corrosion is true?
Corrosion occurs only in the presence of water
What is the process of removing rust from metal surfaces using chemicals or mechanical methods called?
Derusting
What is the process of applying a protective layer of zinc to iron or steel by immersing them in a zinc bath called?
Hot-dip galvanization
What is the process of coating a metal with a layer of another metal using an electric current called?
Electroplating
Which of the following metals is commonly used as a barrier coating to protect steel from corrosion?
Aluminum
Which of the following metals forms a protective oxide layer when exposed to air, preventing further corrosion?
Aluminum
What is the process of removing the oxide layer from a metal surface to expose a clean metal surface called?
Pickling
Which of the following is NOT a method of controlling humidity to prevent corrosion?
Coating with paint
What is the process of coating a metal with a layer of oxide to protect it from corrosion called?
Anodizing
Which of the following is NOT a common method of preventing corrosion in underground pipelines?
Cathodic protection
What is the process of removing impurities or contaminants from a metal surface to prevent corrosion called?
Degreasing
What is the process of converting iron or steel into a stable compound by exposure to air and moisture called?
Rustproofing
Which of the following metals is commonly used as a sacrificial anode for protecting steel structures?
Magnesium
What is the process of forming a protective oxide layer on the surface of aluminum to prevent corrosion called?
Anodizing
Which of the following is NOT a method of chemical treatment to prevent corrosion?
Coating with paint
What is the process of removing the oxide layer from a metal surface by chemical or electrochemical means called?
Derusting
Which of the following is a common method of preventing corrosion in marine environments?
Cathodic protection
What is the process of coating a metal with a layer of zinc using electrolysis called?
Electroplating
Which of the following metals is commonly used as a sacrificial anode in cathodic protection systems?
Zinc
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.