Atomic Structure MCQs with Answers
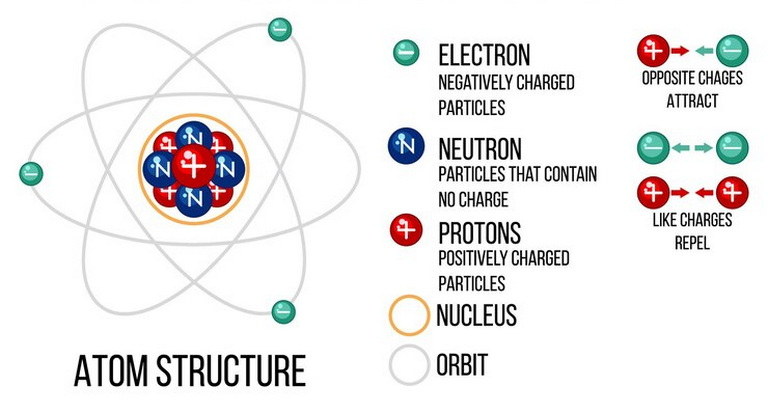
Welcome to the Atomic Structure MCQs with Answers, it helps learners quickly identify areas for improvement in Atomic Structure Online Test.
| Atomic structure is the foundation of chemistry, describing the organization of atoms and their components. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in energy levels or shells. This arrangement forms the basis for understanding atomic models MCQs and Atomic Structure quiz questions in educational settings.
In Atomic Structure MCQs and electron configuration MCQs, students are tested on their knowledge of how electrons occupy specific energy levels around the nucleus. Understanding atomic theory multiple choice questions involves grasping historical and modern models that explain atomic behavior, such as Bohr’s model or the quantum mechanical model. Quantum numbers MCQs delve into the specifics of how electrons are organized within an atom, including their principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number. Mastery of these concepts is crucial in Atomic Structure quiz questions, which assess comprehension of how atomic properties and behaviors are determined by these quantum mechanical principles. |
Atomic Structure Online Quiz
By presenting 3 options to choose from, Atomic Structure Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Atomic Structure Questions and Answers and click over the correct answer.
- Test Name: Atomic Structure MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Atomic Structure Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Atomic Structure Flashcards
What does the Pauli exclusion principle state?
No two electrons can have the same set of quantum numbers
What is the Heisenberg uncertainty principle about?
Limitation on the precision of position and momentum
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.