Welcome to the Reaction Kinetics MCQs with Answers, it helps learners quickly identify areas for improvement in Reaction Kinetics Online Test.
| Reaction kinetics is the branch of chemistry that studies the rates of chemical reactions and the factors that influence them. It explores how quickly reactants are converted into products, providing insights into reaction mechanisms, rate laws, and reaction orders. Understanding reaction kinetics is essential for optimizing industrial processes, designing efficient catalysts, and predicting reaction behavior under various conditions.
For learners and professionals seeking to master this field, Reaction Kinetics MCQs with Answers offer valuable practice. These multiple choice questions cover a wide range of topics, including the determination of reaction orders, calculation of rate constants, and analysis of experimental data to derive rate laws.
Multiple Choice Questions on Reaction Kinetics in quizzes provide practical scenarios where individuals can apply their knowledge to solve problems related to reaction rates and mechanisms. These quizzes often assess understanding of how factors like temperature, concentration, and catalysts affect reaction rates and equilibrium.
A Reaction Kinetics Practice Test allows individuals to assess their comprehension of kinetics principles comprehensively. It helps in identifying strengths and weaknesses before exams, enabling focused revision.
Reaction Kinetics Exam Questions typically require a deeper understanding and application of concepts learned in kinetics, such as interpreting experimental results, predicting reaction behavior, and proposing mechanisms based on kinetic data. Mastering reaction kinetics is essential for advancing both theoretical understanding and practical applications in chemistry. |
Reaction Kinetics Online Quiz
By presenting 3 options to choose from, Reaction Kinetics Quiz which cover a wide range of topics and levels of difficulty, making them adaptable to various learning objectives and preferences. You will have to read all the given answers of Reaction Kinetics Questions and Answers and click over the correct answer.
- Test Name: Reaction Kinetics MCQ Quiz Practice
- Type: Quiz Test
- Total Questions: 40
- Total Marks: 40
- Time: 40 minutes
Note: Answer of the questions will change randomly each time you start the test. Practice each quiz test at least 3 times if you want to secure High Marks. Once you are finished, click the View Results button. If any answer looks wrong to you in Quiz, simply click on question and comment below that question, so that we can update the answer in the quiz section.
Download Certificate of Reaction Kinetics Test
On the end of Quiz, you can download the certificate of the quiz if you got more than 70% marks.
Reaction Kinetics Flashcards

Which of the following statements about the rate of reaction is true?
It decreases over time in all reactions

What does the term 'rate-determining step' refer to in a reaction mechanism?
The slowest step in the mechanism

What is the order of a reaction if its rate law expression is rate = k?

Which of the following statements about activation energy is true?
It is the energy required to initiate a reaction

A reaction has a rate law expression of rate = k[NO]. What is its order with respect to NO?

What is the half-life of a zero-order reaction?
Equal to the initial concentration

Which factor does NOT affect the rate of a reaction?

The overall order of a reaction is determined by ____________.
Adding up the individual orders of reactants in the rate law

A reaction has a rate law expression of rate = k[A][B]. What is its order with respect to A?

What does the term 'rate constant' represent in a chemical reaction?
The proportionality constant relating rate of reaction to concentration of reactants

In a second-order reaction, if the concentration of reactant A is halved, what happens to the rate of the reaction?

Which statement about the rate constant (k) is true?
It is independent of temperature

A reaction has a rate law expression of rate = k[NO]^2. What is its order with respect to NO?

The term 'pre-exponential factor' in the Arrhenius equation represents ____________.
The frequency of collisions between reactant molecules

What is the order of a reaction if its rate law expression is rate = k[A][B]^2?

What is the effect of adding a catalyst on the activation energy of a reaction?
It decreases the activation energy

The rate-determining step in a reaction mechanism is the step that ____________.
Has the highest activation energy

Which of the following expressions represents the rate law for a zero-order reaction?

A reaction has a rate law expression of rate = k[NO2]. What is its order with respect to NO2?

What does a negative value of reaction order indicate?
The reactant inhibits the reaction

The rate constant (k) of a reaction depends on ___________.
Temperature and presence of catalyst

The units of a rate constant (k) for a first-order reaction are:

What is the effect of increasing the concentration of reactants on reaction rate?
It increases the reaction rate

What happens to the rate constant (k) of a reaction when the temperature is increased?

Which of the following is NOT a factor affecting reaction rates?

The collision theory of reaction rates states that _____________.
Reactants must collide with sufficient energy and proper orientation

A reaction has a rate law expression of rate = k[NO]^2[O2]. What is its order with respect to NO?

Which of the following statements about elementary reactions is true?
They involve only one step

The Arrhenius equation relates the rate constant (k) of a reaction to _____________.

Which of the following statements about catalysts is false?
They increase the activation energy

The activation energy of a reaction is the energy difference between ______________.

What is the overall order of a reaction if its rate law expression is rate = k[A]^2[B]?

The half-life of a first-order reaction is independent of the ____________.

For a second-order reaction, the units of the rate constant (k) are:

A reaction that has a rate equation of rate = k[A]^2[B] is ______ order with respect to [A] and _______ order with respect to [B].

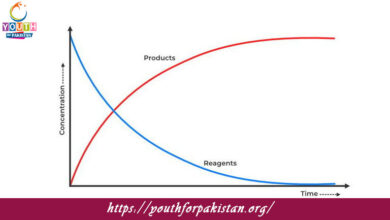
What does the slope of a concentration-time graph of a zero-order reaction represent?

In a first-order reaction, the rate of reaction is directly proportional to ____________.
Concentration of reactant

The rate constant (k) of a reaction increases with __________.

Which of the following factors does not affect reaction rate?

The rate of a chemical reaction depends on _________________.
If you are interested to enhance your knowledge regarding Physics, Computer, and Biology please click on the link of each category, you will be redirected to dedicated website for each category.